Progesterone is a natural steroid hormone which controls women’s reproductive functions . In women, this hormone is produced in the ovaries, the adrenal glands and in the placenta during pregnancy. Progesterone is plays an important role in a woman’s menstrual cycle and it supports pregnancy. It prepares the endometrium for pregnancy in the luteal phase by stimulating proliferation of the endometrium in response to human chorionic gonadotropin (hCG), which is produced by the corpus luteum. In assisted reproduction cycles , the progesterone or hCG levels, or both are low, so the luteal phase needs to be supported with progesterone or with hCG. In Donor egg IVF recipients, exogenous progesterone is used for producing the endometrial luteal phase transformative changes.[1]
A large recent Cochrane review showed that luteal phase support with progesterone is associated with higher rates of live birth and ongoing pregnancy as compared to placebo [2].
Progesterone can be supplemented by oral, vaginal, rectal or parenteral routes. Available progesterone preparations include oral,vaginal, oil-based intramuscular (IM) and the newer preparation aqueous subcutaneous Progesterone .
Oral preparations are of limited use in fertility management because of poor bioavailability [3]. Vaginal progesterone despite lower circulating levels of progesterone achieves adequate endometrial transformation but it is associated with side effects like vaginal discharge and/or local irritation [4,5]. The available intramuscular preparation is oil based and though it achieves adequate serum levels of progesterone, it can cause severe discomfort and pain at the injection site [6]. A new aqueous progesterone preparation for subcutaneous (SC) administration has recently been developed which aims at providing the advantages of existing parenteral preparations without their local tolerability issues, [7,8]. Considering the advantages given by the possibility of self-medication, the SC aqueous formulation can now offer a convenient alternative for patients on ART treatments.
Aqueous progesterone :
Progesterone is a pregnane (C21) steroid and is also known as pregn-4-ene-3,20-dione. Like all unconjugated steroid hormones, progesterone is lipophilic and hydrophobic. Historically this necessitated the administration of progesterone in oil which was traditionally performed per oral or vaginal application in form of suppositories or gel, respectively, or by intra-muscular injection. Because of the lipophilicity of progesterone, commercially available injectable formulations are in the form of dispersions micronized in ethyl oleate. This type of formulations present a number of drawbacks: they are not well tolerated , give rise to irritations at the injection site, are painful and can cause sterile abscess and they cannot be administered subcutaneously. Recently, progesterone has become available as a water-soluble compound for subcutaneous (s.c.) injection . This has been made possible because of the Hydroxy Propyl Beta Cyclodextrin technology.[9]
Cyclodextrins are cyclic hollow structures with a cavity in the centre of the structure. They are bucket shaped oligosaccharides with a hydrophobic cavity and hydrophilic exterior (figure 1). As a result of their molecular structure and shape cyclodextrins have a unique ability to act as molecular containers by entrapping guest molecules in their internal cavity (figure 2).The resulting inclusion complexes are used in a number of applications in pharmaceutical formulations. Hydroxypropyl-B- cyclodextrin is produced by reacting B-cyclodextrin with propylene oxide. Drug is entrapped in the cavity of HPβCD and forms inclusion complexes forming HPβCD-drug complex (host-guest complex) (figure 3)
The use of cyclodextrin to increase the solubility of progesterone in water was first described by Pitha. Hydroxypropyl-β-cyclodextrin (HPBCD) has a high water solubility which allows the solubilization of high quantity of P.[9]Considering a 1:2 guess/host complex stoichiometry it is possible to obtain up to 50 mg/ml of P concentration, which is a considerable dosage for drug development in the progesterone therapy. [9]Once injected under the skin, the progesterone/ cyclodextrin complex easily enters the bloodstream where the cap opens and releases the progesterone . Progesterone remains free in the circulation, as if produced endogenously by the ovaries
Pharmacodynamics – Progesterone is a naturally occurring steroid that is secreted by the ovary, placenta, and adrenal glands. It belongs to a group of steroid hormones called the progestogens,and is the major progestogen in the body. In the presence of adequate estrogen, progesterone transforms a proliferative endometrium into a secretory endometrium . and prepares the uterus for implantation. Progesterone is necessary to increase the endometrial receptivity for implantation of embryo and once implanted it acts to maintain the pregnancy.
Pharmacokinetics- The newly developed water soluble progesterone formulation,
administered as single dose by s.c. and i.m. route, is promptly absorbed, and achieves 3–4 fold higher and prompter progesterone peak serum concentrations compared to the i.m. oil-based formulation. Three studies were conducted to show the pharmacokinetics of the new drug. In 2013, the first study was conducted on aqueous progesterone which was a three-way cross-over, open-label study in 24 postmenopausal women. Comparison of the pharmacokinetic profiles of a single 100-mg dose of aqueous test product administered by subcutaneous (s.c.) and intramuscular (i.m.) injection and an i.m. reference oily product was done . The study demonstrated that the aqueous formulation was bioequivalent to the reference product in terms of extent of exposure.[10] Aqueous formulation (100 mg) was promptly absorbed, achieving progesterone peak serum levels at an earlier time than the reference (1 h vs 7 h; p\0.0001).By one hour post administration of a single dose the mean Cmax was 50.7 +/- 16.3 ng/ml. The progesterone serum concentration decreased following a mono-exponential decay , and by twelve hours post-administration the average concentration was 6.6+/- 1.6ng/ml.The second study was a three-way cross-over open label study of 25, 50 and 100 mg s.c. single doses of the aqueous formulation in 12 post-menopausal women which indicated that the serum pharmacokinetics of progesterone after single ascending s.c. doses of 25, 50 and 100 mg aqueous formulation follow a linear relationship in terms of both Cmax and AUC.[10] The third study, a parallel group, observer-blinded study in 25 fertile women administered multiple subcutaneous 25 and 50 mg doses of the aqueous formulation once daily for 11 days. In the multiple ascending dose study, progesterone concentrations over multiple 25 and 50 mg s.c. injections approached the steady state by day 4 after the start of treatment. Cmax and AUC values at steady state were similar to those obtained after single administration of the same doses and no accumulation of progesterone took place.[10]
Sator et al. had concluded that the Aqueous progesterone formulation showed similar bioavailability as the reference oil-based product . The application of a daily dose of 25 mg s.c. progesterone was equivalent to the physiologic amount produced daily by the ovary in mid-luteal phase and resulted in predecidual changes in 100% of interpretable endometrium samples in a dose finding study . In the preliminary studies, it has been demonstrated that the serum levels of P achieved with 25 mg were above the threshold necessary for pre-decidualization to occur.
The safety profiles of the single and multiple dose treatments did not give rise to any specific concerns and the observed adverse events were consistent with those reported in the literature. As expected, the low progesterone multiple dose (25 mg s.c.) was better tolerated than the high dose (50 mg s.c.), as demonstrated by significantly less adverse effects and less injection site reactions
Discussion
The recent introduction of subcutaneous progesterone for luteal phase support in IVF has broadened the spectrum of treatment options for women undergoing IVF treatment, especially for women who do not tolerate or dislike vaginal formulations.[11] But it is of great importance that the efficacy and safety profile of a new formulation is established in studies of high methodological quality before it is used on a larger scale. Two large phase III trials of similar methodology conducted in a similar patient population has shown that differences between s.c. and vaginal progesterone in pregnancy likelihood are small and indistinguishable.[11]
The first phase III trial conducted by Lockwood et al “Subcutaneous Progesterone Versus Vaginal Progesterone Gel for Luteal Phase Support in Patients Undergoing In-Vitro Fertilization (IVF)” was designed as a prospective, open, randomised, parallel, multicentre, two arm trial. A total of 683 ART patients in 13 European countries were randomized into two groups: Prolutex, 25 mg subcutaneously daily (n = 339); and Crinone, 90 mg 8 % gel daily (n = 344)., Prolutex or Crinone gel was begun on the day of oocyte retrieval for LPS and continued for up to 10 weeks. The nonsignificant difference between the groups was-3.09 %(95 %confidence interval [CI]-9.91 to 3.73), which indicated noninferiority of Prolutex to Crinone. There was no statistically significant differences for any of the other secondary efficacy endpoints like comfort of usage and overall satisfaction. The study concluded that implantation rate, pregnancy rate, live birth rate, and early miscarriage rate for Prolutex were similar to those for Crinone. The adverse event profiles were also similar, and Prolutex was safe and well tolerated .[12]
The second phase III trial conducted by Baker et al entitled “Subcutaneous Progesterone Versus Vaginal Progesterone tablets for Luteal Phase Support in In Vitro Fertilization “ was also a prospective, open, randomized, parallel, multicentre, two-arm trial. 800 women between 18 and 42 years from eight U.S. sites were randomized and they received either progesterone 25 mg s.c. once a day or 100 mg intravaginal twice a day from the day of oocyte retrieval for a maximum of ten weeks depending on presence of pregnancy. Of these, 782 received embryo transfer. Using a PP analysis, which included all patients who received an embryo transfer (Prolutex = 392; Endometrin = 390), the ongoing pregnancy rate per retrieval for subcutaneous versus vaginal progesterone was 41.6 versus 44.4 %, with a difference between groups of -2.8 % (95 % CI -9.7, 4.2). This was consistent with the non-inferiority of subcutaneous progesterone for luteal phase support. In addition, rates of initial positive b-hCG (56.4 % subcutaneous vs. 59.0 % vaginal; 95 % CI -9.5, 4.3), clinical intrauterine pregnancy with fetal cardiac activity (42.6 vs. 46.4 %; 95 % CI -10.8, 3.2), implantation rate (33.2 vs. 35.1 %; 95 % CI-7.6, 4.0), live birth (41.1 vs. 43.1 %; 95 % CI -8.9, 4.9), and take-home baby (41.1 vs. 42.6 %; 95 % CI -8.4, 5.4) were also comparable.[13] Both formulations were well tolerated and there was no difference in serious adverse events. Baker et al. concluded that subcutaneous progesterone represents a novel option for luteal phase support in women undergoing IVF who prefer not to use a vaginal preparation for personal reasons or who wish to avoid the side effects of vaginal or IM routes of administration.[13]
Conclusion
To conclude, there is enough evidence that the new aqueous P preparation available for SC administration is effective at priming the endometrial changes seen in the menstrual cycle in the absence of endogenous progesterone. SC injections are easy to perform, causes less pain than IM injection and also avoids the messy discharges reported with vaginal administration that many women complain of. The possibility of administering progesterone subcutaneously for luteal phase support in IVF cycles has broadened the spectrum of options which is an advantage for women disliking vaginal treatments for cultural or personal reasons.
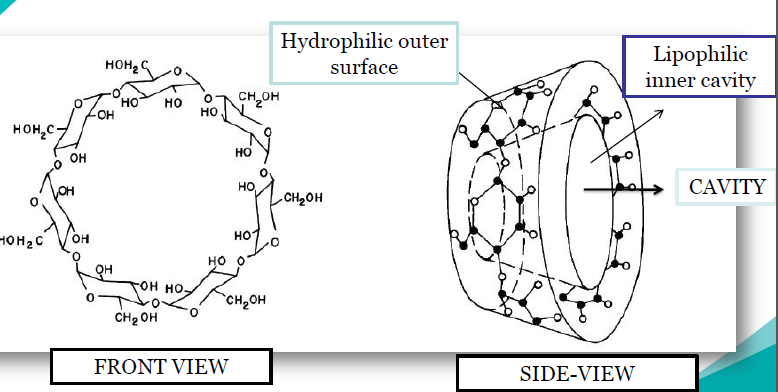
Figure: 1
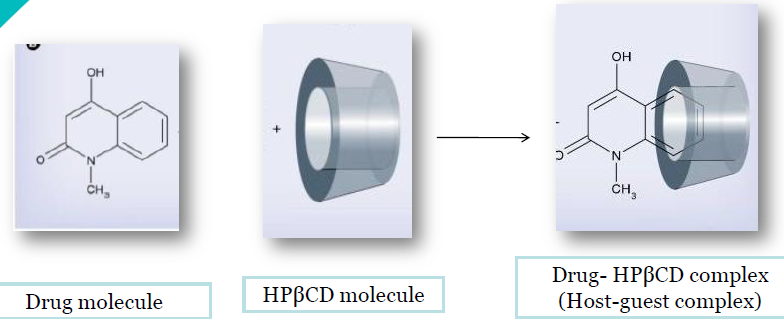
Figure 2
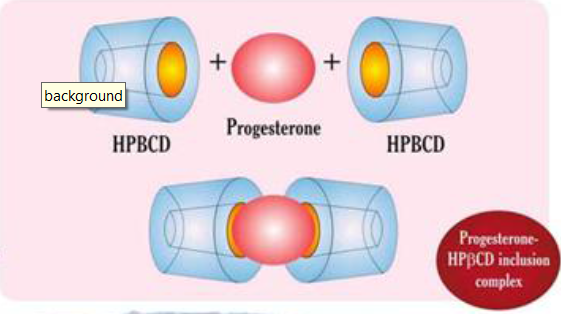
Figure 3
References
1.Gautam N. Allahbadia ,Has ART Finally Got a Patient-Friendly Progesterone? The Journal of Obstetrics and Gynecology of India 2015;
2. van der Linden M, Buckingham K, Farquhar C, et al. Luteal phase support for assisted reproduction cycles. Cochrane Database Syst Rev. 20115;(10):CD009154. doi: 10.1002/14651858. CD009154.pub2.
3.Simon JA, Robinson DE, Andrews MC, et al. The absorption of oral micronized progesterone: the effect of food, dose proportionality, and comparison with intramuscular progesterone. Fertil Steril. 1993;60:26–33.
4.Blake EJ, Norris PM, Dorfman SF, et al. Single and multidose pharmacokinetic study of a vaginal micronized progesterone
insert (Endometrin) compared with vaginal gel in healthy reproductive aged female subjects. Fertil Steril. 2010;94:1296–301.
5.Levy T, Yairi Y, Bar-Hava I, et al. Pharmacokinetics of the progesterone-containing vaginal tablet and its use in assisted
reproduction. Steroids. 2000;65:645–9.
6. Nillius SJ, Johansson ED. Plasma levels of progesterone after vaginal, rectal, or intramuscular administration of progesterone.
Am J Obstet Gynecol. 1971;110:470–7.
7. Zoppetti G, Puppini N, Ospitali F, et al. Pharmaceutics, preformulation and drug delivery. J Pharmaceut Sci. 2007;97:1729–36.
8.Zoppetti G, Puppini N, Pizzuti M, et al. Water soluble progesterone- hydroxypropyl-b-cyclodextrin complex for injectable
formulations. J Incl Phenom Macrocycl Chem. 2007;57:283–8.
9 G. Zoppetti ,N. Puppini, M. Pizzutti , A. Fini, T. Giovani , S. Comini , Water soluble progesterone–hydroxypropyl-b-cyclodextrin complex for injectable formulations; J Incl Phenom Macrocycl Chem (2007) 57:283–288
10. Michael Sator, Milko Radicioni, Barbara Cometti et al. Pharmacokinetics and safety profile of a novel progesterone aqueous formulation administered by the s.c. route. Gynecological Endocrinology, 2013; 29(3): 205–208
11. Doblinger J, Cometti B, Trevisan S, Griesinger G (2016) Subcutaneous Progesterone Is Effective and Safe for Luteal Phase Support in IVF: An Individual Patient Data Meta-Analysis of the Phase III Trials. PLoS ONE 11(3): e0151388. doi:10.1371/journal.pone.0151388
12 . Lockwood G, Griesinger G, Cometti B. Subcutaneous progesterone versus vaginal progesterone gel
for luteal phase support in in vitro fertilization: a noninferiority randomized controlled study. Fertil Steril.
2014 Jan; 101(1):112–119.e3. doi: 10.1016/j.fertnstert.2013.09.010 PMID: 24140033
13.Baker VL, Jones CA, Doody K, Foulk R, Yee B, Adamson GD, et al. A randomized, controlled trial comparing the efficacy and safety of aqueous subcutaneous progesterone with vaginal progesterone for
luteal phase support of in vitro fertilization. Hum Reprod. 2014 Oct 10; 29(10):2212–20. doi: 10.1093/
humrep/deu194 PMID: 25100106
